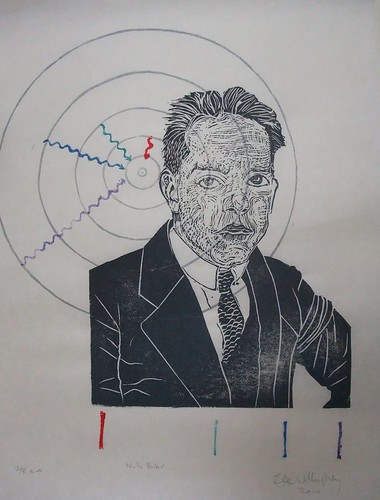
This is a block printed portrait of Danish physicist Niels Bohr (1885-1962), subject of the Mad Scientists of Etsy's November Challenge. Bohr is an obvious choice - the Nobel laureate not only was one of the central figures of 20th century quantum mechanics, godfather of the Copenhagen interpretation, he was known for his generousity, open-mindedness, and clear ethics (which cannot convincingly be said of all of his contemporaries). He advocated sharing knowledge and the peaceful use of nuclear energy. He was also known for taking long walks and thinking out-loud. I often wondered about what Mrs. Bohr thought, faced with his incessant rambling on the interpretation of quantum mechanics.
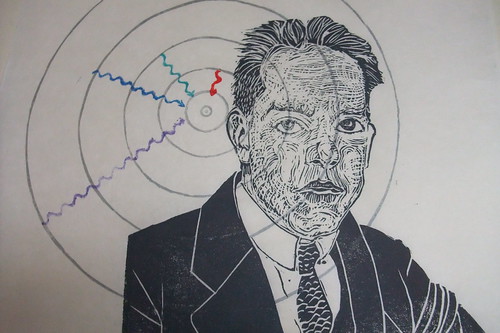
One of his most famous contributions to quantum mechanics was the Bohr-Rutherford model of the atom. In the print, Bohr is shown in front of the Bohr model of the Hydrogen atom (all the concentric circles are actually at the appropriate spacing, proportional to the n the orbit number squared*). Physicist knew that the atoms were neutral, and yet positive charge was highly localized, in what we now know as the nucleus, from the Rutherford Gold-Foil Experiment. As is traditional in science, this experiment isn't named after those who performed it (Geiger - yes, as in Geiger counter - and Marsden) but after their supervisor, Lord Ernest Rutherford of Nelson. They shot alpha particles (Helium nuclei: 2 protons and 2 neutrons, but all they knew was that they were small and had charge +2) at a very thin sheet of gold foil. Gold can be pounded into sheets which are scarcely a few atoms thick. Anyway, some of the alpha particles bounced right back, which Rutherford famously described thus,
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive center, carrying a charge.[2]
So Rutherford proposed his model of the atom by making analogy to the solar system. He postulated that the positive nucleus is like the sun, with negative electrons orbiting like planets. This model seems to have reasonated with the public (and you still see absurb, outdated images to symbolize atomic physics, with a dot and various elipses around it) - but it's wrong. You see, it suffers from ...wait for it... spiral death! Physicists are a melodramatic bunch. You see, accelerating charges, like electrons going in circles, give off light, hence loose energy, so would be expected to spiral into the nucleus and annihilate.

Bohr proposed that the orbits of electrons were somewhat like planetary orbits (though circular, and at specific quantized distances). To explain how orbitting charged electrons didn't lose energy he stipulated that perhaps they simply weren't allowed anywhere but the specific orbits. They could lower their energy state if excited by falling to a lower orbit, giving off a specific photon of a specific colour related to the specific, quantized, difference between energy levels. This also explained how the spectra of gases had distinct, thin, spectral lines. I've illustrated this with the Balmer series - because it is composed of lines which are visible to the eye (H-alpha is red and caused by a jump from the 3rd to 2nd orbit; H-beta is cyan and caused by a jump from the 4th to 2nd orbit; H-gamma is indigo and caused by a jump from the 5th to 2nd orbit; and H-delta is violet and caused by a jump from the 6th to 2nd orbit). I've shown both the quantum jumps (squigelly arrows - squigelly lines are traditional for photons) and by the line spectrum below Bohr.
This is a first edition print (one of eight) on Japanese kozo (mulberry) paper, (12.5" by 17").
*The fact that I needed to make my concentric circles at appropriately spaced radii (and the line spectra are at the right spacing too, of course) probably reflects on my sanity in some way, but you know, how can I resist the Bohr Hydrogen atom? I mean one can actually solve it analytically from first principles! How cool is that? Even though, in theory, one ought to be able to continue with this logic and derive all of chemistry as a simple application of quantum mechanics, it very rapidly becomes too complicated and we must rely on computers.









No comments:
Post a Comment