 |
| Ada, Countess Lovelace, 3rd edition linocut by Ele Willoughby |
This year I'm participating in an entire group art show celebrating Ada Lovelace Day. The Art.Science.Gallery show Go Ahead and Do It: Portraits of Women in STEM culminates today! I will share all of my portraits of women in science (and links to where I tell their stories) below.
 |
| Marie Skłodowska-Curie, linocut with glow-in-the-dark ink by Ele Willoughby, 2014 |
In previous years, I've specifically avoided writing about Marie Curie because she is often the one historical figure people can name. I don't like to do the obvious thing and particularly want to highlight the under appreciated heroines of science. However the result is that her truly remarkable achievements haven't been celebrated here, just because of her fame. So, with a collection of portraits and stories written on the less well known, today I'll write about the well-known and why she in fact deserves her fame.
Marie Skłodowska-Curie (7 November 1867 – 4 July 1934), Polish-born, naturalized-French physicist and chemist, as the first woman to win a Nobel prize, the only woman to ever win TWO Nobel prizes, and the only person ever to win in two different sciences: physics and chemistry! She was also the first female professor at the University of Paris, and in 1995 became the first woman to be entombed on her own merits in the Panthéon in Paris. Born Maria Salomea Skłodowska in Warsaw, she studied secretly at the Floating University there before moving to Paris where she earned higher scientific degrees, met her PhD supervisor and future husband Pierre.
She was one of the pioneers who helped explain radioactivity, a term she coined. She was the one who first developed a means of isolating radioacitve isotopes and discovered not one, but two new elements: polonium (named for her native country) and radium. She also pioneered radioactive medicine, proposing the treatment of tumors with radioactivity. She founded medical research centres, the Curie Institutes in Paris and Warsaw which are still active today. She created the first field radiology centres during World War I. Each one of these achievements alone would warrant being memorialized in the annals of science and medicine; she did all of these things. She died in 1934 from aplastic anemia brought on by exposure to radiation, including carrying test tubes of radium in her pockets during research and her World War I service in her mobile X-ray units.
Her pioneering work explaining radioactivity earned her the 1903 Nobel Prize in Physics with her husband Pierre Curie and with physicist Henri Becquerel. At first, the Committee intended to honour only Pierre and Becquerel, but Swedish mathematician Magnus Gösta Mittag-Leffler, an advocate of women in science, alerted Pierre to the situation. (You may recall that it was the same man who helped Sofia Kovalevski secure a University position in Stockholm and that she collaborated on works of literature and had what was called a "romantic friendship" with his sister Duchess Anne-Charlotte Edgren-Leffler). After Pierre's complaint, Marie's name was added to the nomination. The 1911 Nobel Prize in Chemistry was awarded to her "in recognition of her services to the advancement of chemistry by the discovery of the elements radium and polonium, by the isolation of radium and the study of the nature and compounds of this remarkable element."
Her life and legacy are truly extraordinary!
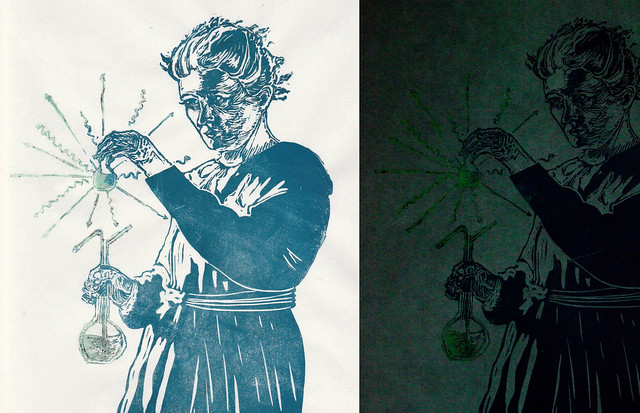 |
| Marie Skłodowska-Curie, linocut with glow-in-the-dark ink show in the light and dark by Ele Willoughby, 2014 |
Not only was her work original and providing revolutionary insight on the theoretical side at the time, but the sheer heroic dedication and labour involved in her experimental work cannot be overstated. Having recognized that pitchblende ore must contain multiple elements which were giving off radiation, she and Pierre were able to show in 1898 that two new elements Polonium and Radium were needed to explain their observations. They then sought to actually isolate these elements. From a ton of pitchblende, she separated one-tenth of a gram of radium chloride in 1902. In 1910 Marie Curie isolated pure radium metal - a full 12 years after she and Pierre published their preliminary evidence for its existence. This involved working in a shed, meticulously separating the radioactive material from the inert and then dividing the radioactive material into its various sources for many years - all the while raising their young daughter when not at the lab.
Both of the elements she discovered are radioactive, meaning that they spontaneously give off radiation. All of the isotopes of polonium emit alpha particles, but Polonium-210 will emit a blue glow which is caused by excitation of surrounding air. Radium emits alpha, beta and gamma particles - that is 2 protons and 2 neutrons, electrons as well as x-rays. Thus, I've shown her sample surrounded by the symbols of these particles: the straight and wiggly lined arrows for the massive particles and high-energy light photons or gamma rays respectively, and made the sample with glow-in-the-dark ink. While the materials she discovered and worked with would have glowed due to radioactivity, never fear... these prints glow due to phosphorescence - a different process which is not dangerous. The ink will absorb UV light (for instance, from sunlight) and re-emit it in the dark.
The linocut is printed on Japanese kozo paper 9.25" by 12.5" (23.5 cm by 32 cm) in an edition of eight.
You will soon find links to my previous Ada Lovelace Day posts and other short bios and portraits of heroines of science in my next post.

No comments:
Post a Comment